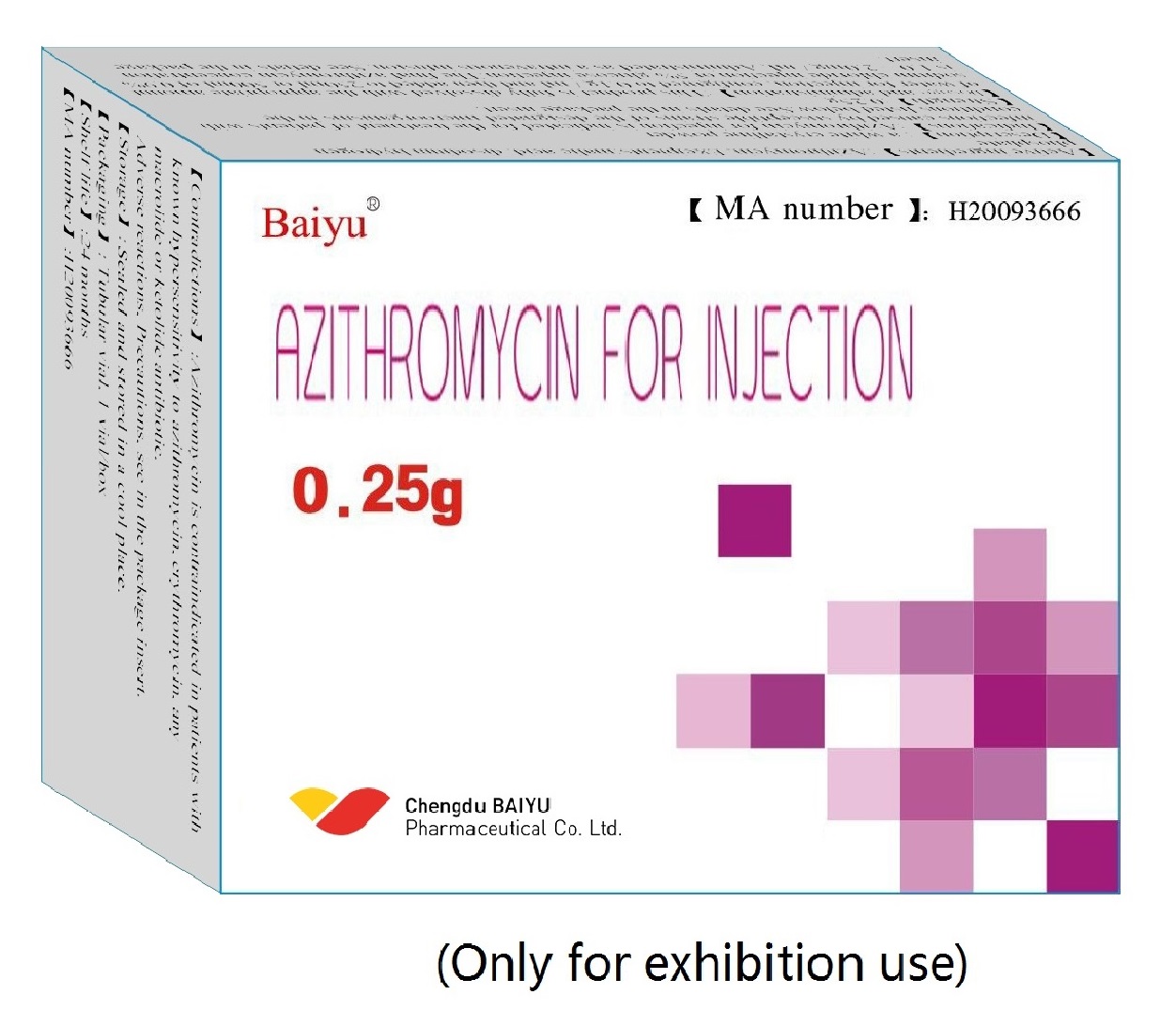
Azithromycin for injection
Generic name:Azithromycin for Injection
Active ingredient:Azithromycin
Excipient:Malic acid, mannitol and disodium hydrogen phosphate
Description:White or off-whiteporous mass or powder.
Indication:This product is suitable for the following infections caused by sensitive pathogenic strains:
1. Community-acquired pneumonia (CAP)caused by Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae, Staphylococcus aureus, or Streptococcus pneumoniae and requiresa first intravenous infusion treatment.
2. Pelvic inflammatory disease caused by chlamydia trachomatis, Neisseria gonorrhoeaeand Mycoplasma hominis and requiresa first intravenous infusion treatment.
Strength
0.25g (250,000 units)
0.5g (500,000 units)
Dosage and administration
Administration
Prior to use, add some water for injection into the vial to dissolve the content completely to obtain a solution having a concentration of 0.1 g/ml, and transfer the dissolved solution intoa 250 ml or 500 ml of 0.9% sodium chloride injection or 5% glucose injection to obtain a solution with a final azithromycin concentration of 1.0 to 2.0 mg/ml, then followed by the intravenous infusion. The whole infusion period should be controlled at 3 hours for 1.0 mg/ml and 1 hour for 2.0 mg/ml. Intravenous infusion period should be controlled not less than 60 minutes, and the concentration of not exceed 2.0mg/ml.
Dosage
1.CAP: 0.5g per time for adults, once a day, at least 2 consecutive days, then followed by changing to oral preparations of azithromycin of 0.5g per day, the course of treatment is 7-10 days. Changing time for oral treatment should be determined by the physician based on the clinical response.
2.Pelvic inflammatory disease: 0.5g per time for adults, once a day, after one or two days of medication, followed by changing to oral preparations of azithromycin of 0.25g per day, the course of treatment is 7 days. Changing time for oral treatment should be determined by the physician based on the clinical response.
Storage
Sealed, kept in a cool and dry place.
Packaging
Unopened glass vials
10vials/box, 1 vial/box
Shelf life
24months
Specification
ChP (2015)
Approval number
GUOYAOZHUNZI H20093666
GUOYAOZHUNZIH20093667
Active ingredient:Azithromycin
Excipient:Malic acid, mannitol and disodium hydrogen phosphate
Description:White or off-whiteporous mass or powder.
Indication:This product is suitable for the following infections caused by sensitive pathogenic strains:
1. Community-acquired pneumonia (CAP)caused by Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae, Staphylococcus aureus, or Streptococcus pneumoniae and requiresa first intravenous infusion treatment.
2. Pelvic inflammatory disease caused by chlamydia trachomatis, Neisseria gonorrhoeaeand Mycoplasma hominis and requiresa first intravenous infusion treatment.
Strength
0.25g (250,000 units)
0.5g (500,000 units)
Dosage and administration
Administration
Prior to use, add some water for injection into the vial to dissolve the content completely to obtain a solution having a concentration of 0.1 g/ml, and transfer the dissolved solution intoa 250 ml or 500 ml of 0.9% sodium chloride injection or 5% glucose injection to obtain a solution with a final azithromycin concentration of 1.0 to 2.0 mg/ml, then followed by the intravenous infusion. The whole infusion period should be controlled at 3 hours for 1.0 mg/ml and 1 hour for 2.0 mg/ml. Intravenous infusion period should be controlled not less than 60 minutes, and the concentration of not exceed 2.0mg/ml.
Dosage
1.CAP: 0.5g per time for adults, once a day, at least 2 consecutive days, then followed by changing to oral preparations of azithromycin of 0.5g per day, the course of treatment is 7-10 days. Changing time for oral treatment should be determined by the physician based on the clinical response.
2.Pelvic inflammatory disease: 0.5g per time for adults, once a day, after one or two days of medication, followed by changing to oral preparations of azithromycin of 0.25g per day, the course of treatment is 7 days. Changing time for oral treatment should be determined by the physician based on the clinical response.
Storage
Sealed, kept in a cool and dry place.
Packaging
Unopened glass vials
10vials/box, 1 vial/box
Shelf life
24months
Specification
ChP (2015)
Approval number
GUOYAOZHUNZI H20093666
GUOYAOZHUNZIH20093667